Articles of Interest
JournalDoc is proud to work with a team of experts who understand the challenges faced by Healthcare Professionals and pleased to offer a weekly article of interest representing trends and new information affecting the study and practice of medicine.
Origin: Original Investigation Article Published in the Journal of the American Medical Association (JAMA) on October 24/31, 2023
Original Article Title: Traditional Chinese Medicine Compound (Tongxinluo) and Clinical Outcomes of Patients With Acute Myocardial Infarction
Tongxinluo and Acute Myocardial Infarction
What is Tongxinluo?
Tongxinluo, a revered formula from the realm of Traditional Chinese Medicine (TCM), enjoys widespread acclaim across China and parts of Asia for its potential heart health benefits. Employed to combat coronary artery disease, angina, and the aftereffects of strokes, it is credited with enhancing blood flow, diminishing the thickness of blood, and fortifying against the formation of arterial plaques. This concoction blends a diverse array of components, such as herbs and animal extracts, each chosen for their unique contributions inline with TCM philosophies.
The precise mix of ingredients in Tongxinluo might show slight variations among different producers, but it typically encompasses elements like ginseng, leech, centipede, scorpion, and a variety of herbs. This blend aims to tackle the intricate web of causes that TCM identifies as underlying cardiovascular disorders, including the stagnation of qi or vital energy, the stasis of blood, and the imbalance of yin and yang forces within the body.
Although there exists a body of scientific research pointing to Tongxinluo’s positive impact on heart health, for instance, easing angina symptoms and lowering risk indicators for cardiovascular conditions—the evidence remains inconclusive. Further, rigorously conducted studies are essential to ascertain its true effectiveness and how it works. Therefore, individuals are advised to seek medical advice before incorporating Tongxinluo into their regimen, particularly due to potential interactions with other drugs and considerations for individual health conditions.
What Is Interesting About This Article?
This article presents a fascinating exploration into the realm of TCM, which stands as the most frequently utilized therapeutic agent on a global scale. With an impressive repertoire of approximately 12,800 traditional Chinese medicines, the majority of these compounds are sourced from plant-based materials, or from intricate blends incorporating animal and mineral elements. A prime example of such a compound is Tongxinluo, a TCM crafted from a carefully curated mixture of both plants and animal products.
The significance of this study lies in its findings regarding the efficacy of Tongxinluo, particularly observed at the 30-day and 1-year marks post-treatment. The research underscores a noteworthy clinical improvement in patients who were administered Tongxinluo, highlighting its potential as a beneficial therapeutic option within the vast and diverse spectrum of traditional Chinese medicines. This study not only contributes to our understanding of TCM’s role in modern healthcare but also opens avenues for further research into the mechanisms and broader applications of Tongxinluo in treating various conditions.
JournalDoc Notes
The intersection between TCM and the rigorous methodologies characteristic of Western medical research remains a sparsely explored territory. However, this particular study, conducted by Chinese researchers, stands out as a commendable effort to bridge this gap, adhering to the stringent criteria of evidence-based medicine through the use of randomized controlled trials (RCTs). This approach not only lends credibility to the findings but also paves the way for a more integrative global healthcare perspective.
Dr. Richard Bach, in his insightful editorial, draws attention to the unconventional yet intriguing composition of Tongxinluo, which includes such ingredients as the dried bodies of cockroaches, centipedes, leeches, cicadas, and scorpions. While at first glance, the constituents may seem unconventional to those accustomed to Western pharmacology, they underscore the rich diversity and complexity inherent in TCM formulations.
The study’s revelation that the primary adverse reactions associated with Tongxinluo were stomach discomfort and nausea is not entirely unexpected, given the nature of its components. Nonetheless, the reported relative risk reduction of 37% for adverse cardiovascular outcomes following Tongxinluo treatment is both striking and compelling. Such a significant finding not only highlights the potential therapeutic benefits of Tongxinluo but also underscores the need for further rigorous research.
The impressive outcomes of this study about Tongxinluo and acute myocardial infarction underscore the importance of conducting more randomized controlled trials to explore the efficacy and safety of traditional Chinese medicines. By doing so, the medical community can better understand how these ancient practices can be harmonized with modern medical standards, ultimately leading to enhanced patient care and a broader array of treatment options.
CLICK HERE FOR MORE INFORMATION ON THIS STUDY
JournalDoc is an innovative platform designed to support medical professionals and healthcare administrators in making daily decisions. Leveraging a combination of patented algorithms, expertise from medical professionals, and artificial intelligence (AI), JournalDoc efficiently searches through accredited and reputable databases. This process ensures the provision of reliable, reputable, and the most relevant medical information based on queries, facilitating better informed decision-making especially in compels clinical scenarios.
Disclaimer: Articles of Interest selections by JournalDoc physicians are independent, unbiased and have no commercial conflict of interest. JournalDoc neither supports nor endorses the findings or opinions expressed in the article. Findings in the article may need to be supported by further research and/or the consensus of experts.
CTS-AMI Investigators:
Yuejin Yang, MD, PhD
Xiangdong Li, MD, PhD
Guihao Chen, MD, PhD
Runlin Gao, MD
Eric D. Peterson, MD, MPH
Yaling Han, MD, PhD
Boli Zhang, MD
Ying Xian, MD, PhD
Haitao Zhang, MD, PhD
Yuan Wu, MD
Yanmin Yang, MD
Jianhua Wu, MD
Chuntong Wang, MD
Shenghu He, MD
Zhong Wang, MD
Yixin Wang, MD
Zhifang Wang, MD
Hui Liu, MD
Xiping Wang, MD
Minzhou Zhang, MD
Jun Zhang, MD, PhD
Jia Li, MD
Tao An, MD
Hao Guan, MD
Lin Li, MD
Meixia Shang, MD
Chen Yao, MD
Jun Zhang, MD, PhD (Listed twice, assuming leadership role or different individuals with same name and title)
Author Affiliations
Origin: Original Investigation Article Published in The New England Journal of Medicine (NEJM) Original on February 9, 2024
Original Article Title: An Oral Interleukin-23–Receptor Antagonist Peptide for Plaque Psoriasis
What is the Interleukin-23–Receptor?
The interleukin-23 receptor (IL-23R) is a type I cytokine receptor, encoded by the IL23R gene in humans. It functions alongside the interleukin-12 receptor β1 subunit (IL-12Rβ1) and is activated by interleukin 23 (IL-23). The gene transcript for IL23R spans 2.8 kilobases, containing 12 exons that translate into a 629 amino acid protein. This protein is a type I transmembrane entity featuring a signal peptide, an N-terminal fibronectin III-like domain, and an intracellular segment with three potential tyrosine phosphorylation sites. Notably, in mitogen-activated lymphocytes, there are 24 splice variants of IL23R. Variability in the IL23R sequence, particularly in the single-nucleotide polymorphisms within the domain that binds IL-23, can affect Th17 cell activation across individuals. Additionally, a soluble form of IL-23R, comprising only the extracellular domain, acts as a decoy receptor for IL-23. This soluble receptor competes with the membrane-bound form, influencing the regulation of the Th17 immune response and thus impacting inflammation and immune function.
What is an Antagonist Peptide?
Antagonists peptides represent an emerging class of biologically active agents that exhibit unique properties. These antagonists, which are selective for specific receptor classes, serve as valuable tools in uncovering both the physiological and pathological functions of endogenous peptides and their associated receptors.
What is Interesting about this Article?
The advent of monoclonal antibodies has revolutionized the management of various immune-mediated inflammatory conditions, such as psoriasis. Traditionally, these monoclonal antibodies are large protein molecules that necessitate administration through injection, which can often be a barrier to treatment due to patient discomfort or the need for clinical administration. In contrast, the development of a new, orally administered drug marks a significant advancement in treatment convenience and patient compliance. Clinical trials have demonstrated that this orally taken medication is more effective than a placebo, offering a promising new avenue for the treatment of patients suffering from these challenging diseases. This shift not only enhances the efficacy of treatment but also significantly improves the quality of life for patients by simplifying the administration process.
JournalDoc Notes
The study involved data from a multicenter, randomized, dose-finding trial that included 255 patients suffering from moderate-to-severe plaque psoriasis. These patients were randomly assigned to undergo treatment using varying dosages of a novel, orally administered peptide. This peptide functions as an interleukin-23-receptor antagonist, effectively blocking the signaling of interleukin-23 and the production of downstream cytokines, which are crucial in the inflammatory process of psoriasis. In the trial, some patients received a placebo. The treatment period lasted for 16 weeks, allowing researchers to observe and compare the efficacy and safety of different dosages of the peptide in alleviating the symptoms of plaque psoriasis against the placebo control. This trial aims to establish a foundational understanding of the optimal dosing regimen, while assessing the potential benefits and risks associated with this innovative therapeutic approach.
The development of an orally administered psoriasis drug represents a significant breakthrough for patients, providing a much-needed alternative to the more traditional, invasive methods of treatment. In a related editorial by Dr. Joel M. Gelfand, advancements in bioengineering are highlighted as pivotal in enabling the oral delivery of large, complex protein-based drugs. Semiglutide is cited as a prime example of this innovation, showcasing the potential of new drug delivery methods. The particular study in question concentrated on the drug’s impact on skin symptoms associated with psoriasis. However, it remains unclear how this orally administered drug might affect other organs or related disorders such as psoriatic arthritis. This underscores the necessity for further research to fully understand the broader implications and potential side effects of the treatment on overall health, beyond its immediate effects on psoriatic skin lesions.
CLICK HERE FOR MORE INFORMATION ON THIS STUDY
JournalDoc is a cutting-edge platform tailored to assist medical professionals and healthcare administrators in their daily decision-making processes. It integrates patented algorithms, insights from medical experts, and advanced artificial intelligence (AI) to thoroughly search through accredited and trusted databases. This sophisticated approach guarantees the delivery of dependable and highly relevant medical information tailored to specific queries. By providing such critical data, JournalDoc enhances informed decision support, proving particularly valuable in complex clinical situations.
Disclaimer: Articles of Interest selections by JournalDoc physicians are independent, unbiased and have no commercial conflict of interest. JournalDoc neither supports nor endorses the findings or opinions expressed in the article. Findings in the article may need to be supported by further research and/or the consensus of experts.
Origin: The New England Journal of Medicine, January 4, 2024
Original Article Title: Drug-Eluting Resorbable Scaffold versus Angioplasty for Infrapopliteal Artery Disease
What is a Drug-Eluting Resorbable Scaffold?
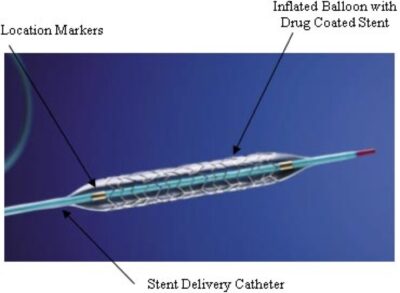
Drug-Eluting Resorbable Scaffolds (DERS) represent a significant advancement over traditional metal stents. Constructed from biodegradable materials, DERSs serve the critical function of maintaining arterial patency but do so temporarily. Over time, they dissolve or are metabolized by the body, which can diminish the long-term risks commonly associated with permanent stents.
The composition of DERS involves bioresorbable polymers that allow them to integrate seamlessly with bodily processes, eventually disappearing once their structural support is no longer necessary. Additionally, these scaffolds are imbued with pharmacological agents, similar to traditional drug-eluting stents. These medications are released gradually to prevent restenosis, which is the re-narrowing of the artery due to cell proliferation at the treatment site.
This technology underscores a pivotal shift towards interventions that not only provide immediate therapeutic benefits but also align with the body’s natural healing mechanisms, enhancing long-term cardiovascular health outcomes.
What is Angioplasty for Infrapopliteal Artery Disease?
This minimally invasive procedure targets the arteries below the knee, which are crucial for blood supply to the lower leg and foot. In cases of peripheral artery disease (PAD), these arteries may become narrowed or blocked, leading to severe complications such as chronic pain, ulcers, and even the risk of limb amputation.
The angioplasty procedure for infrapopliteal artery disease involves several key steps. Initially, access is gained through a small incision, typically in the groin area, to introduce a catheter into the artery. This catheter is then meticulously guided to the site of the blockage. Upon reaching the target area, a small balloon attached to the tip of the catheter is inflated. This inflation compresses the plaque against the artery walls, effectively widening the artery and restoring essential blood flow.
In certain scenarios, a stent—a small wire mesh tube—may also be deployed to keep the artery open. While stenting is a common practice in the treatment of coronary and carotid artery diseases, its application in infrapopliteal arteries is considered more selectively. The decision to place a stent depends on the patient’s specific condition and the severity of the arterial blockage.
This procedure not only mitigates the immediate risks associated with PAD but also plays a crucial role in the long-term management of the disease, aiming to improve patient quality of life and prevent more severe outcomes.
What is Interesting about this Article?
Peripheral artery disease (PAD) remains a pressing health issue globally, affecting approximately 230 million people worldwide, including between 7 to 12 million in the United States. PAD leads to the narrowing or blockage of arteries, escalating to chronic limb-threatening ischemia—a critical condition that could culminate in tissue loss or amputation if not effectively addressed. Angioplasty traditionally has been utilized to manage such severe arterial conditions. However, this treatment is frequently accompanied by the need for repeated interventions due to complications such as restenosis, where the artery narrows once more after treatment.
In response to these ongoing challenges, innovative treatments have been developed to enhance patient outcomes. One notable advancement is the introduction of the drug-eluting resorbable scaffold, a device designed to temporarily support the artery while releasing everolimus, a drug that inhibits restenosis. A pivotal study demonstrated that this approach is superior to traditional angioplasty for patients with infrapopliteal artery disease. The scaffold provides structural support until the artery can sustain itself and gradually dissolves within the body, minimizing the risk of the long-term complications often associated with permanent stents. These results signify a substantial progression in PAD treatment, offering a more effective and potentially safer alternative to conventional angioplasty, particularly for those facing chronic limb-threatening scenarios.
JournalDoc Notes
Untreated limb-threatening ischemia in the legs presents a severe health challenge, marked by persistent pain, a high incidence of ulceration, gangrene, and a substantial risk of amputation. These dire consequences highlight the need for effective intervention strategies. In an insightful editorial, Dr. Joshua A. Beckman suggests that the management of PAD is evolving significantly with the advent of device therapy, particularly through the use of resorbable scaffolds. These innovative devices are designed to provide temporary support to the vessel wall, crucially preventing both acute and subacute vessel closures, thereby advancing the treatment landscape of PAD.
This evolving approach was the focus of a recent study that examined not only the mechanical success of the device but also the clinical outcomes for the patients. Such research underscores the fundamental principles of evidence-based medicine, which relies heavily on tangible clinical benefits to validate medical practices. The study’s emphasis on both device efficacy and patient-centric outcomes serves as a robust model for future research. It suggests that subsequent studies in this field should continue to integrate clinical outcomes to fully assess the effectiveness and safety of new technologies in real-world scenarios, ensuring that advancements in medical devices translate into meaningful health benefits for patients with PAD.
CLICK HERE FOR MORE INFORMATION ON THIS STUDY
Disclaimer: The Article of the Week selections made by JournalDoc physicians are independent and unbiased, with no commercial conflicts of interest. JournalDoc neither supports nor endorses the opinions or findings presented in the articles. The conclusions drawn in these articles may require further validation through additional research and expert consensus.
JAMA Original Investigation
Journal of the American Medical Association, January 2, 2024
Data from the SURMOUNT-4 Randomized Clinical Trial, a phase 3 open label withdrawal trial with 670 participants at 70 sites in 4 countries. Participants received once weekly subcutaneous injections of Tirzepatide for 36 weeks and then half were randomized to continue receiving drug or placebo for 52 weeks.
What’s Interesting about this article?
- Tirzepatide is a single molecule that combines glucose -dependent insulintropic polypeptide (GIP) and GLP-1 receptor agonism resulting in synergistic effects on appetite, food intake and metabolism.
- Tirzepatide was recently approved in the U.S. by the FDA, marketed as ZepboundTM.
- Withdrawing Tirzepatide resulted in substantial regain of lost weight, whereas continued treatment maintained and augmented weight reduction.
JournalDoc Comments:
- These findings are consistent with other studies that found that patients must remain on the new weight loss drugs or substantial weight gain occurs.
- The requirement for long term use of Tirzepatide is worrisome because of loss of muscle and GI adverse effects reported with this class of drugs.
- Until the need for maintenance therapy is solved, the new weight loss drugs should only be prescribed for patients with obesity and either/or diabetes or cardiovascular disorders.
CLICK HERE FOR MORE INFORMATION ON THIS STUDY
Disclaimer: Article of the Week selections by JournalDoc physicians are independent, unbiased and have no commercial conflict of interest. JournalDoc does not support or endorse the findings or opinions expressed in the article. Findings in the article may need to be supported by further research and/or the consensus of experts.
NEJM Original Article
New England Journal of Medicine, January 18, 2024
Data from a subtrial of an RCT that assessed the cardiovascular safety of testosterone treatment in 5204 men ages 45 to 80 years of age with preexisting or high risk of cardiovascular disease and one or more symptoms of hypogonadism and low testosterone levels. Participants were randomly assigned to apply a testosterone or placebo gel daily, and then asked at each visit if they had had a fracture since the previous visit. The median follow-up was 3.19 years.
What’s Interesting about this article?
- Testosterone treatment in men with hypogonadism improves bone density and quality. However, there is no data on incidence of fractures with extended use in a large sample of middle-aged or older men.
- Numerically, there was a higher incidence of fractures in men who received testosterone in this study.
- In the original study of this group of men, there was no difference in adverse cardiovascular events in those that received testosterone rather than placebo.
JournalDoc Comments:
- The increase in incidence of fractures in the testosterone group was a surprising result.
- The increase in fracture risk was not large – 3.50% in the testosterone group versus 2.46% in the placebo group and most fractures were associated with trauma rather than osteoporosis.
- However, in an accompanying editorial by Mathis Grossmann, MD, Ph.D and Bradley Anawalt, the authors recommended that older men with obesity and low testosterone should be considered at higher risk of fracture if prescribed testosterone therapy.
CLICK HERE FOR MORE INFORMATION ON THIS STUDY
Disclaimer: Article of the Week selections by JournalDoc physicians are independent, unbiased and have no commercial conflict of interest. JournalDoc does not support or endorse the findings or opinions expressed in the article. Findings in the article may need to be supported by further research and/or the consensus of experts.