Origin: Original Investigation Article Published in The New England Journal of Medicine (NEJM) Original on February 9, 2024
Original Article Title: An Oral Interleukin-23–Receptor Antagonist Peptide for Plaque Psoriasis
What is the Interleukin-23–Receptor?
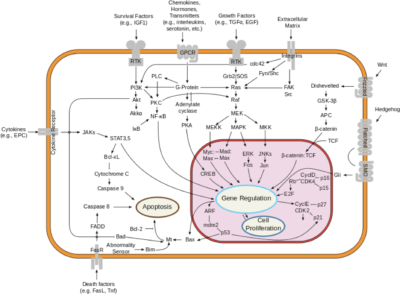
The interleukin-23 receptor (IL-23R) is a type I cytokine receptor, encoded by the IL23R gene in humans. It functions alongside the interleukin-12 receptor β1 subunit (IL-12Rβ1) and is activated by interleukin 23 (IL-23). The gene transcript for IL23R spans 2.8 kilobases, containing 12 exons that translate into a 629 amino acid protein. This protein is a type I transmembrane entity featuring a signal peptide, an N-terminal fibronectin III-like domain, and an intracellular segment with three potential tyrosine phosphorylation sites. Notably, in mitogen-activated lymphocytes, there are 24 splice variants of IL23R. Variability in the IL23R sequence, particularly in the single-nucleotide polymorphisms within the domain that binds IL-23, can affect Th17 cell activation across individuals. Additionally, a soluble form of IL-23R, comprising only the extracellular domain, acts as a decoy receptor for IL-23. This soluble receptor competes with the membrane-bound form, influencing the regulation of the Th17 immune response and thus impacting inflammation and immune function.
What is an Antagonist Peptide?
Antagonists peptides represent an emerging class of biologically active agents that exhibit unique properties. These antagonists, which are selective for specific receptor classes, serve as valuable tools in uncovering both the physiological and pathological functions of endogenous peptides and their associated receptors.
What is Interesting about this Article?
The advent of monoclonal antibodies has revolutionized the management of various immune-mediated inflammatory conditions, such as psoriasis. Traditionally, these monoclonal antibodies are large protein molecules that necessitate administration through injection, which can often be a barrier to treatment due to patient discomfort or the need for clinical administration. In contrast, the development of a new, orally administered drug marks a significant advancement in treatment convenience and patient compliance. Clinical trials have demonstrated that this orally taken medication is more effective than a placebo, offering a promising new avenue for the treatment of patients suffering from these challenging diseases. This shift not only enhances the efficacy of treatment but also significantly improves the quality of life for patients by simplifying the administration process.
JournalDoc Notes
The study involved data from a multicenter, randomized, dose-finding trial that included 255 patients suffering from moderate-to-severe plaque psoriasis. These patients were randomly assigned to undergo treatment using varying dosages of a novel, orally administered peptide. This peptide functions as an interleukin-23-receptor antagonist, effectively blocking the signaling of interleukin-23 and the production of downstream cytokines, which are crucial in the inflammatory process of psoriasis. In the trial, some patients received a placebo. The treatment period lasted for 16 weeks, allowing researchers to observe and compare the efficacy and safety of different dosages of the peptide in alleviating the symptoms of plaque psoriasis against the placebo control. This trial aims to establish a foundational understanding of the optimal dosing regimen, while assessing the potential benefits and risks associated with this innovative therapeutic approach.
The development of an orally administered psoriasis drug represents a significant breakthrough for patients, providing a much-needed alternative to the more traditional, invasive methods of treatment. In a related editorial by Dr. Joel M. Gelfand, advancements in bioengineering are highlighted as pivotal in enabling the oral delivery of large, complex protein-based drugs. Semiglutide is cited as a prime example of this innovation, showcasing the potential of new drug delivery methods. The particular study in question concentrated on the drug’s impact on skin symptoms associated with psoriasis. However, it remains unclear how this orally administered drug might affect other organs or related disorders such as psoriatic arthritis. This underscores the necessity for further research to fully understand the broader implications and potential side effects of the treatment on overall health, beyond its immediate effects on psoriatic skin lesions.
CLICK HERE FOR MORE INFORMATION ON THIS STUDY
JournalDoc is a cutting-edge platform tailored to assist medical professionals and healthcare administrators in their daily decision-making processes. It integrates patented algorithms, insights from medical experts, and advanced artificial intelligence (AI) to thoroughly search through accredited and trusted databases. This sophisticated approach guarantees the delivery of dependable and highly relevant medical information tailored to specific queries. By providing such critical data, JournalDoc enhances informed decision support, proving particularly valuable in complex clinical situations.
Disclaimer: Articles of Interest selections by JournalDoc physicians are independent, unbiased and have no commercial conflict of interest. JournalDoc neither supports nor endorses the findings or opinions expressed in the article. Findings in the article may need to be supported by further research and/or the consensus of experts.
